By David T. Rubin, MD, Professor of Medicine, University of Chicago Medicine
Dear Colleagues,
Here is my understanding of the different stages through which SARS-CoV-2 becomes COVID-19. I also added some thoughts on how we can prevent these stages and treat patients.
– Stage 1: Exposure to SARS-CoV-2
- This occurs via close contact to known or unknown carriers. Currently, SARS-CoV-2 is thought to be in the “community”.
- Prevention of this stage is avoiding exposure by staying at home, practicing meticulous hand hygiene, and isolating those who are sick.
– Stage 2a: Entry of SARS-CoV-2 into the body
- SARS-CoV-2 mostly enters through the mouth and respiratory tract, presumably via inhalation of aerosolized viral particles.
- But there is increasing evidence of gut involvement and possible “fecal-oral” mode of transmission.1
– Stage 2b: Entry of SARS-CoV-2 into human cells
- This appears to occur mostly via Angiotensin Converting Enzyme 2 (ACE2) receptors on cells which allow the virus in.
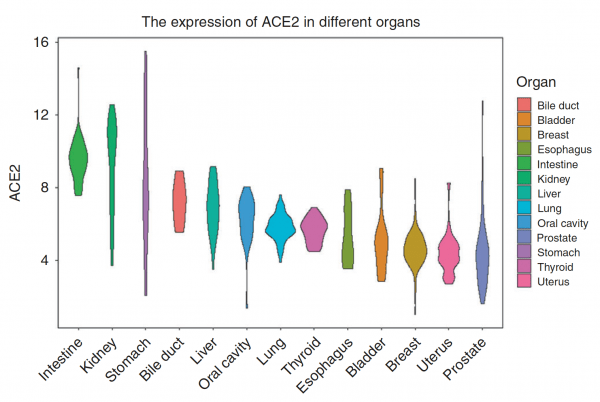
- Expression of ACE2 receptor is higher in the GI tract (including the mouth) than in the lung.2
You might be wondering, just as I was, that if there are so many more receptors in the gut, why is the primary manifestation of COVID-19 in the lungs? It might be just where most of the viral exposure occurs.
How can we prevent Stage 2b?
- Could we down regulate ACE2 expression? Block these receptors with anti-ACE2 antibodies or protease inhibitors? That is work underway.3,4
– Stage 3: Inflammatory response by the body as a reaction to SARS-CoV-2 invasion – this is when the infection becomes the disease (COVID-19)
- This inflammatory response by the body’s immune system causes the fever and symptoms experienced by patients and ultimately leads to tissue damage. (This primarily affects the lungs but can affect other tissues as well).
How do we treat COVID-19?
- Treatment of the inflammation to reduce damage (anti-IL6, anti-TNF, but not steroids).5,6
- Anti-virals to reduce the viral load and let the body “catch up”.7,8
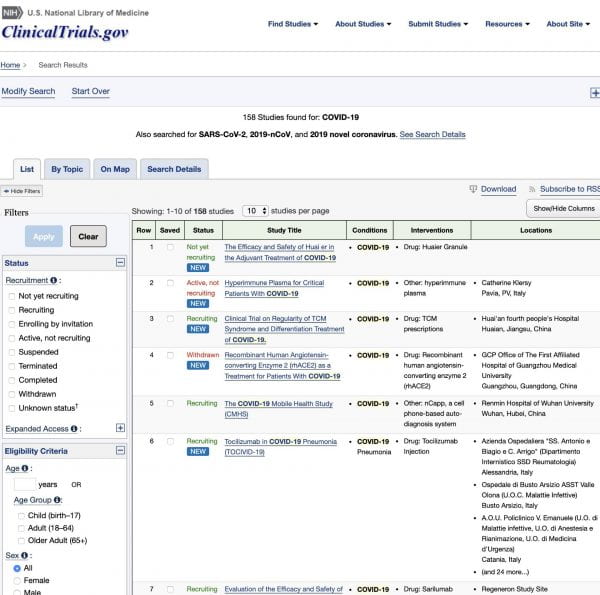
- There are currently more than 150 ongoing clinical trials on COVID-19!9
Vaccine trials are under way10,11, but this will take some time. That’s why we must continue all efforts to flatten the curve, support our healthcare workers, and treat the sick. We will get there.
Thoughts and comments are welcome.
Best wishes,
David
David T. Rubin, MD
Professor of Medicine, University of Chicago Medicine
References and useful reads:
- Hindson, J. COVID-19: faecal–oral transmission?. Nat Rev Gastroenterol Hepatol (2020). https://doi.org/10.1038/s41575-020-0295-7
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi:10.1038/s41368-020-0074-x
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet Lond Engl. 2020;395(10224):565-574. doi:10.1016/S0140-6736(20)30251-8
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. March 2020. doi:10.1016/j.cell.2020.02.052
- Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2). doi:10.23812/CONTI-E
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473-475. doi:10.1016/S0140-6736(20)30317-2
- Mitjà O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health. 2020;0(0). doi:10.1016/S2214-109X(20)30114-5
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
- Search of: COVID-19 – List Results – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=&cntry=&state=&city=&dist=. Accessed March 25, 2020.
- NIH clinical trial of investigational vaccine for COVID-19 begins. National Institutes of Health (NIH). https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins. Published March 16, 2020. Accessed March 25, 2020.
- Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) to Prevent SARS-CoV-2 Infection – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04283461. Accessed March 25, 2020.