SARS Vaccine and IBD Tweetorial Summary from December 1
David T. Rubin
Here are my thoughts and a few updates about SARS-CoV-2 vaccines, and also about important implications for our IBD patients.
Development of multiple SARS-CoV-2 vaccines in the timeframe we have seen is a monumental scientific achievement. Necessity literally bred invention.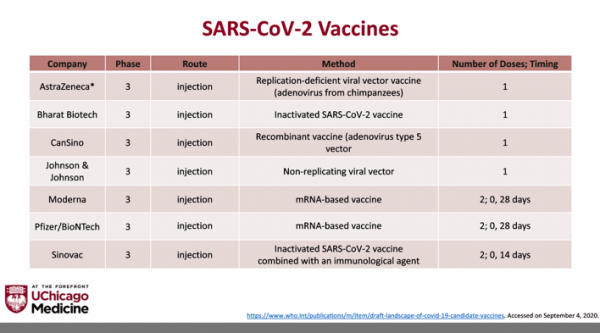
The Pfizer and Moderna vaccines are messenger RNA vaccines. They use genetic material to trigger the body’s immune system to make antibodies against the spike proteins on the surface of SARS-CoV-2. They DO NOT contain actual viral particles and can’t cause infection.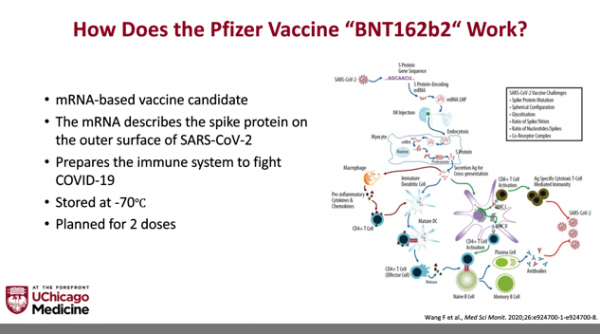
Two doses are required with these vaccines. This is not uncommon for vaccines. The first exposes the immune system to the antigen, the second will activate memory cells, so your body will remember how to protect you if it gets exposed to SARS-CoV-2.
The reason for the cold temperature is to stabilize the messenger RNA so it maintains its structural integrity and then can do its job in your body. This is a well-described but surmountable logistical challenge.
The vaccines will come with temperature monitors so you will know if it has been kept at the right temp during transport.
I am told that the FDA is reviewing the Pfizer vaccine on 10 Dec and the Moderna on 13 Dec. The CDC has committed to rapid review and updated recommendations within 24 hours of the FDA decisions.
First in line for the vaccine will be front line healthcare workers. Also, key will be residents of long-term care facilities. The CDC Advisory Committee on Immunization Practices (ACIP) voted today on this stratification. https://www.cnn.com/2020/12/01/health/cdc-acip-covid-19-vaccine-recommendation-vote/index.html
The CDC Advisory Committee on Immunization Practices (ACIP) has previously outlined plans for vaccination. Technically, our IBD folks will be in the second tranche under the category of “use of immune weakening medicines”.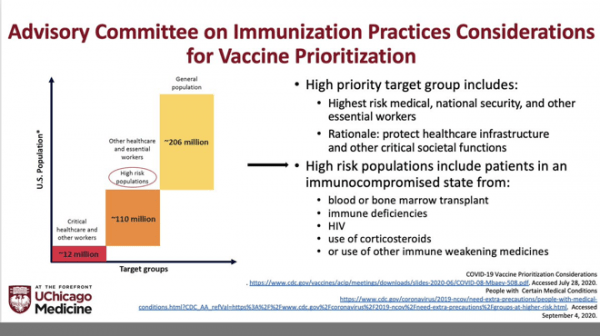
Reminder that IBD patients have not been at increased risk for SARS-CoV-2 infection or COVID-19, and that the immune therapies used in IBD (except steroids) have not been associated with worse outcomes. There may even be some protection while on these meds. (Brenner EJ et al., Gastroenterology. [Epub ahead of print May 18 2020].
Key questions: Will IBD patients on immune therapies be less likely to develop immunity? Does it matter which therapy you are receiving whether you will develop immunity? MAYBE?
There are some data that older vaccines like for pneumococcal pneumonia (PSV-23) or influenza are less likely to result in immunity if patients are on combined anti-TNF and immunomodulators (azathioprine or 6-MP). (Mamula P et al., Glin Castroenterol Hepatol. 2007;5(7):851-856).
But it’s variable! Some patients are more immunogenic than others. With vaccines, being immunogenic is a good thing (you are more likely to respond to the vaccine). With monoclonal antibodies, being immunogenic is not a good thing (you become “immune” to the therapy).
So what about this messenger RNA vaccine? I’ve discussed a bit with experts here. Seems that anti-TNF (inflix, adalim) and anti-IL12/23 (ustekinumab) and possibly JAKinib (tofacitinib) will not impair immunity to these new vaccines.
Unclear whether cellular trafficking inhibitors (vedolizumab) may impair since they may affect lymphocytes ability to respond to the virus in the gut. Thiopurines (azathioprine, 6-MP) (which affect lymphocytes) may impair immunity.
5-ASAs (mesalamine), antibiotics will not affect immunity to the vaccine. But also important is the potency and “immunogenicity” of the vaccine itself, which may overcome any effect of these therapies and be effective anyway.
Example is the modern herpes zoster (shingles) recombinant vaccine (Shingrix) which is quite potent and effective at inducing immunity even in patients who are receiving chemotherapy. If you want to read more, look at this great study: Vink P et al., Cancer. 2019: 1301-1312.
I believe that these vaccines against SARS-CoV-2 will be appropriate and safe for our patients who are on immune therapies. I do not know that they will be AS effective at inducing immunity, but I suspect based on descriptions that they will come very close to being so.
There will be 3 years of f/u from the current vaccine trials. There will be post-marketing surveillance in the general population and the ACIP will adjust their recommendations. But it is clear that we need as a society to work together with good leadership to get this done.
I will get vaccinated. I will work hard to get my patients vaccinated. In the meantime, everyone please stay safe and be vigilant. Protect each other.
